Sickle cell anemia is an inherited genetic disorder caused by a point mutation (SNP) in the hemoglobin-producing gene. This mutation changes the shape of one of the cell’s proteins, which results in sickle-shaped hemoglobin (hence the name). This different hemoglobin, in addition to not having the same capacity as the original to transport oxygen, is also more rigid and has a greater tendency to clump together and obstruct blood vessels (especially the narrower ones), causing constant pain, anemia, swelling of the hands and feet and increasing the risk of infections and stroke.
The first symptoms of the disease begin to appear at five or six months of age. That is, they do not happen during pregnancy or even in the first months after birth. The reason for this is that fetuses and newborns have a different predominant hemoglobin in their blood: fetal hemoglobin (known as F).
The difference between fetal and adult hemoglobin is that the first is made up of a pair of proteins known as alpha and a pair of gamma proteins, while the second associates a pair of alphas with a pair of betas. Their format and function are very similar though, especially when compared to sickle cell disease’s altered version.
It turns out that the gene that controls the production of fetal hemoglobin is not the same as the one that gives the instructions for producing the adult form. What happens is that the gene responsible for the fetal form is deactivated when the gene for the adult form begins to take control. And that’s when symptoms begin in people who have two copies of the pathogenic variant.
The standard treatment for sickle cell disease today is the use of medications such as hydroxyurea, which results in an increase in the proportion of fetal hemoglobin in the blood. However, not all patients benefit from the use of this medication and the exact mechanism of action of this drug is still unknown, unlike the side effects, which are well documented.
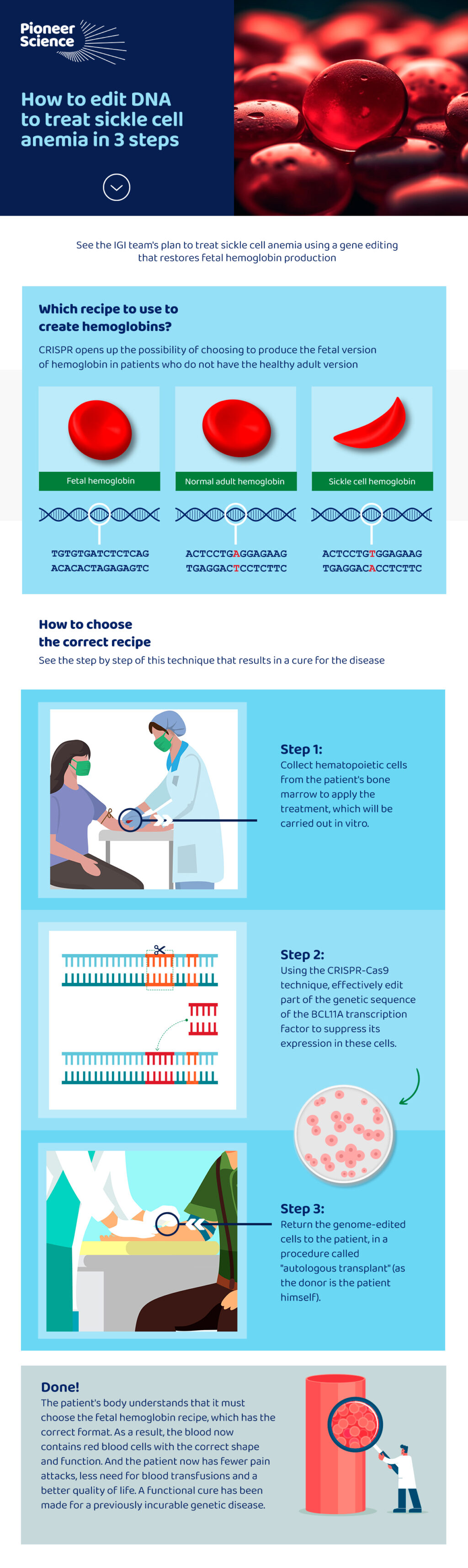
This is why IGI’s proposal can be useful, as mentioned by fellow Bruno Solano in this interview. He works with the biochemist who won the 2020 Nobel Prize in Chemistry, Jennifer Doudna. Dr. Doudna won the Nobel Prize precisely for discovering the CRISPR technique, which allows editing the genome and paves the way for the cure of genetic diseases. Bruno is one of the fellows who are training with Doudna’s team through the partnership with Pioneer Science. The key lies in hematopoietic stem cells, those that are located in the bone marrow and give rise to all other blood cells.
The technique, which has already been analyzed in other studies with mice and humans, consists of suppressing the genetic transcription factor known as BCL11A. As BCL11A is responsible for the suppression of the gene that codes for fetal hemoglobin proteins, in practice, they start to be produced again, which can result in a significant improvement or even a functional cure for the disease, once the patient starts to having a greater proportion of red blood cells with normal geometry in the blood, minimizing the risk of thrombosis and other risks associated with sickle cell hemoglobin.
See the infographic below for a step-by-step guide to this solution:
Share